Design of AMP
Overview
In this project, fusion antimicrobial peptides + narrow-spectrum antibiotics are used as the main bactericidal means. Considering the high bactericidal performance, high targeting, low toxicity of the vector, and environmental stability, we selected the fusion peptide + Temporin-PTa8L (a narrow-spectrum antibiotic) composed of a specific targeting domain and a broad-spectrum AMP domain as the bactericidal module of the drug.
Fusion Peptide
The study found that Staphylococcus aureus's pheromones fused with plectasin to selectively kill Staphylococcus aureus1. We synthesized a parental peptide (CASYFCRWWWLL-NH2) by attaching pheromone-removing thiolactone macrocyclic to short AMP. At the same time, the addition of disulfide bonds to the target domain can enhance the targeting activity against Staphylococcus aureus2.
The targeted antimicrobial activity of antimicrobial peptides depends not only on the targeting domain, but also on the killing domain. Therefore, we have designed a series of killing domains against Staphylococcus aureus for the following reasons:
(1) Tryptophan (W) has a unique ability to interact with membranes, which helps peptides anchor on the surface of bacteria, and the π configuration of tryptophan-rich short peptide WWW motifs is essential for targeted killing MRSA3;
(2) Hydrophobicity is closely related to antimicrobial activity and cell selectivity. AMPs with appropriate hydrophobicity can disrupt membrane structure and cause cytolysis4;
Eventually, AMP S2 was born5. It is a fusion peptide consisting of a species-specific targeting domain and a broad-spectrum AMP domain. In the current study, S2 has specific killing activity against Staphylococcus aureus and produces little resistance compared to penicillin. At the same time, S2 also has excellent salt tolerance and biocompatibility. Because leucine and less positive charge kill gram-positive bacteria more effectively, S2 exhibits stronger targeted antimicrobial capabilities against Staphylococcus aureus.
Narrow Spectrum Antibiotics
According to the relevant literature, it can be seen that the best peptide in the literature is Temporin-PTa8L6.
Temporin-PTa8L——LLGSLLKLLPKLL
However, the application to the intestinal environment still needs in vitro experiments to obtain the most suitable antimicrobial peptides, please click the experiment for detailed design.
Final Sequence
For the subsequent identification and purification of the protein, we added a 6×His tag.
HHHHHHLLGSLLKLLPKLLCASYFCRWWWLL-NH2(C14-C19)
Discussion
1.The target structure of S2 is the π structure of www, and the wide hydrophobic surface of leucine is the target structure of Temporin-PTa8L, and this connection does not destroy the structure of the two.
2.Because it will be applied to the large intestine, trypsin acts on the peptide bond composed of the basic amino acids arginine and leucine, and the intestinal peptidase acts on the carboxyl terminus of Asp-Asp-Asp-Lys. Comparing the above sequences, it can be seen that there is no action site
3.The pH in the large intestine is about 8.3, and the above peptide can be roughly calculated according to the isoelectric point of amino acids to obtain 8.3, which is slightly higher than the isoelectric point of the peptide, making the peptide slightly negative. However, in the literature, surface MRSA deploys a positively charged part on the cell surface, and increasing the positively charged residue does not improve the potency of the peptide, but decreases the cell selectivity. We believe that this peptide theoretically has the ability to exert efficacy in the large intestine environment, but it still needs to be verified by experiments and adjusted in time (if there are problems such as too short and unable to translate)
Design of AMP circuit
Overview
Staphylococcus aureus widely has agr two-component systems to achieve information sensing, which secretes AIP as a pheromone small protein, and after the extracellular AIPs bind to the transmembrane protein AgrC, the quorum sensing signal will gradually phosphorylate the aspartate residue of the intracellular protein AgrA. We used this principle to sense the concentration of Staphylococcus aureus in the environment.
At the same time, considering the need to take into account the expression efficiency and concentration control, we used the lysogenic pathway-related pathway proteins of bacteriophages, including cI, cII, cIII, cro and other series of proteins, to introduce excellent and complete feedback control in the AMP synthesis system, including positive feedback initiation of concentration response of Staphylococcus aureus and negative feedback regulation of maximum concentration containment of AMP. Next, we will elaborate.
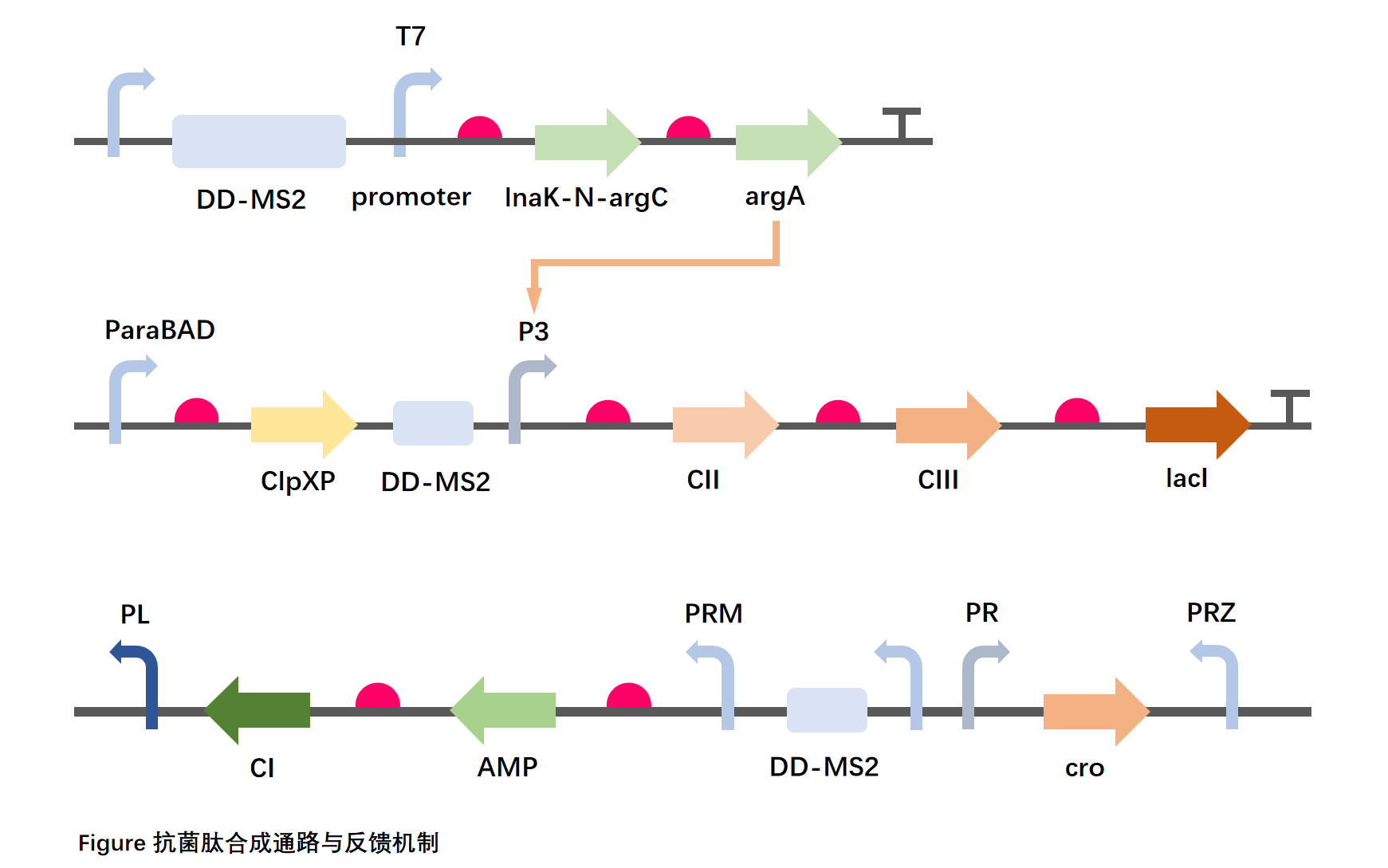
Design
In order to sense the concentration of Staphylococcus aureus in the environment, we introduced an Agr-structured functional module composed of agrC and agrA expressed under the T7 promoter in chassis organisms for AIP signal sensing and intracellular transmission, and introduced the P3 promoter as a switch for the AMP synthesis control module. Considering that AgrC protein may require more accurate membrane surface display, we will try to construct a fusion protein between the truncated functional sequence (lnaK-N) of E. coli surface localization peptide and AgrC to obtain better membrane localization effect.
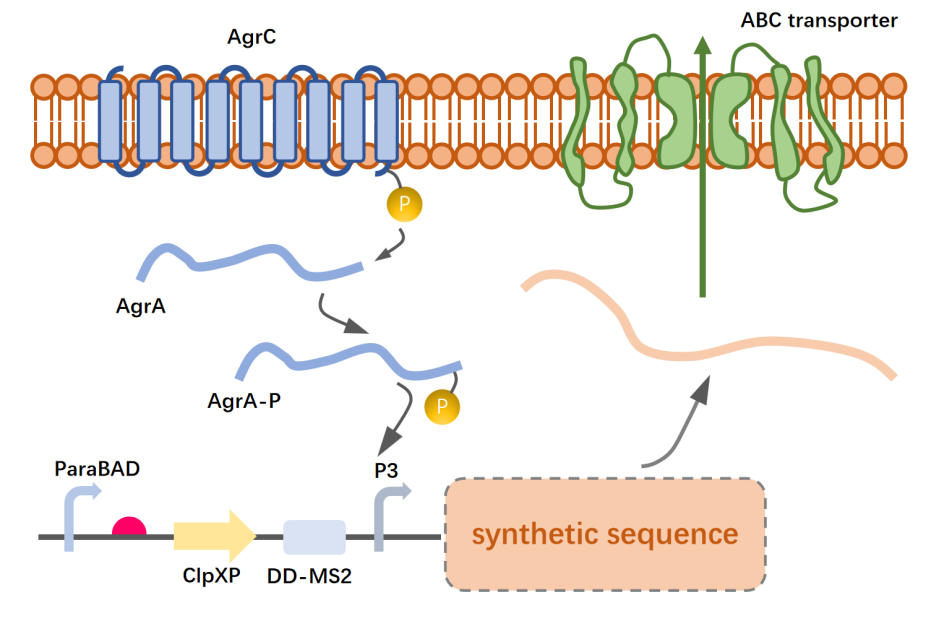
In order to balance expression efficiency and concentration control, we will delete the virulence proteins in the lysogenic pathway pathway of the phage, and then move part of the pathway sequence into the chassis organism, including the promoter PRM encoding the cI gene and the promoter PR and PL with cI binding sites upstream. PR encodes the protein cro7, which has an inverted cII-activated PRZ promoter downstream of the cro. AMP is expressed under the same promoter (PRM) as the cI protein, and the expression of the cII protein and cIII protein is controlled by the P3 promoter. The iGEM parts library BBa_K2036027 provide a lot of useful help for the design of our bioblocks.
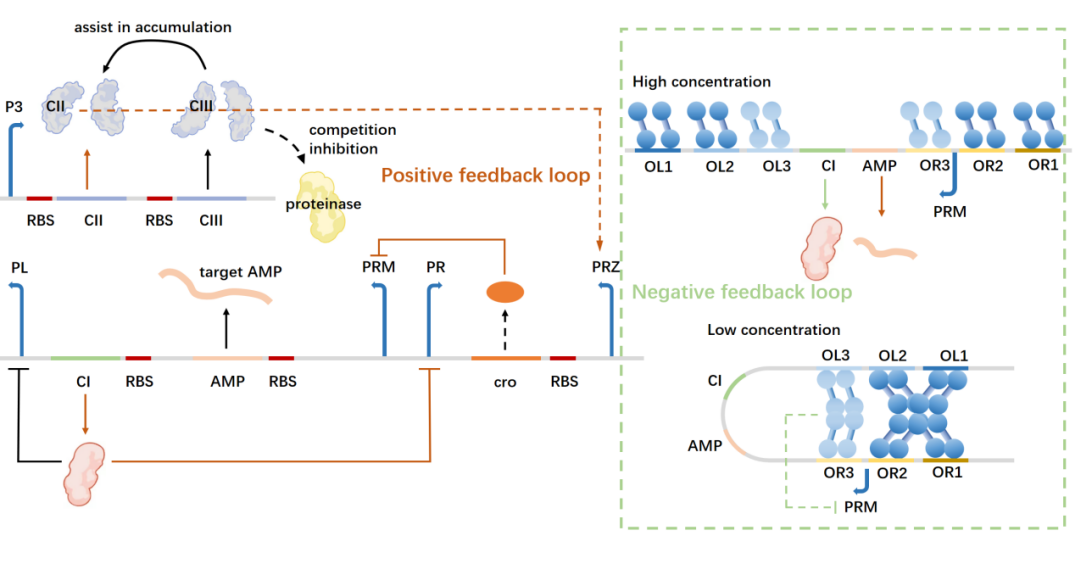
Discussion
Since 9 proteins (AgrC, AgrA, cI, cII, cIII, cro, AMP, CIpXP, LacI) are involved in the synthesis feedback pathway, the pathway size reaches 6655 bp, making it difficult to transfect the plasmid that is too large.
Design of biofilm
Overview
Colonization of Staphylococcus aureus precedes infection8 and opportunistically infects the host. Normally, after entering the intestinal environment in a plankton state, Staphylococcus aureus attaches to the intestinal surface, colonizes and initially forms a biofilm, and then spreads after the biofilm matures, and continues to multiply and expand the chance of infection. Probiotics play an important role in the composition of the gut microbiota, inhibiting the colonization of pathogenic bacteria in the gut and helping the host to establish a healthy protective layer of the intestinal mucosa9. In order to further strengthen the protective effect of engineered probiotics, we introduced hydrogel expression genes10 and secreted hydrogels to promote the production of mucin, the main component of mucus, thereby repairing the intestinal mucosal barrier and enhancing intestinal immunity. Next, we will elaborate.
Design
Referring to previous studies, based on the trilobal factor (TFF) of the fibrous matrix of the intestinal mucosa itself, a cytokine11 that protects the intestinal mucosa, we designed a triplet chimeric protein, the frizzling protein CsgA, tag 6× His for ligation and examination, and the cytokine TFF, It was expected that TFF would be tethered to a crimped fiber matrix to construct a self-crimping hydrogel.
To achieve successful modification and secretion of chimeric proteins, we designed TFF to be placed behind the operon of frizzling protein expression along with the necessary modification genes (csgB, csgC, csgE, csgF, and csgG).
Discussion
Due to the large molecular weight of the hydrogel produced, it will not be absorbed by the human body and will not stay in the intestine for a long time. Its security is enough to be guaranteed.
Probiotics themselves are resistant to the colonization of pathogenic bacteria, and at the same time, they also have a considerable effect on intestinal repair. Our addition of hydrogel genes can further enhance its protective effect.
Reference
- Xi, D.; Teng, D.; Wang, X.; Mao, R.; Yang, Y.; Xiang, W.; Wang, J., Design, expression and characterization of the hybrid antimicrobial peptide LHP7, connected by a flexible linker, against Staphylococcus and Streptococcus. Process Biochemistry 2013, 48 (3), 453-461.
- de Leeuw, E.; Burks, S. R.; Li, X.; Kao, J. P. Y.; Lu, W., Structure-dependent functional properties of human defensin 5. FEBS Lett 2007, 581 (3), 515-520.
- Zarena, D.; Mishra, B.; Lushnikova, T.; Wang, F.; Wang, G., The π Configuration of the WWW Motif of a Short Trp-Rich Peptide Is Critical for Targeting Bacterial Membranes, Disrupting Preformed Biofilms, and Killing Methicillin-Resistant Staphylococcus aureus. Biochemistry 2017, 56 (31), 4039-4043.
- Yang, Z.; He, S.; Wang, J.; Yang, Y.; Zhang, L.; Li, Y.; Shan, A., Rational Design of Short Peptide Variants by Using Kunitzin-RE, an Amphibian-Derived Bioactivity Peptide, for Acquired Potent Broad-Spectrum Antimicrobial and Improved Therapeutic Potential of Commensalism Coinfection of Pathogens. J Med Chem 2019, 62 (9), 4586-4605.
- Shang, L.; Li, J.; Song, C.; Nina, Z.; Li, Q.; Chou, S.; Wang, Z.; Shan, A., Hybrid Antimicrobial Peptide Targeting Staphylococcus aureus and Displaying Anti-infective Activity in a Murine Model. Front Microbiol 2020, 11, 1767.
- Mishra, B.; Wang, G. J. J. o. t. A. C. S., Ab initio design of potent anti-MRSA peptides based on database filtering technology. 2012, 134 (30), 12426-9.
- Lee, S.; Lewis, D. E. A.; Adhya, S., The Developmental Switch in Bacteriophage λ: A Critical Role of the Cro Protein. Journal of Molecular Biology 2018, 430 (1), 58-68.
- Kluytmans, J.; van Belkum, A.; Verbrugh, H., Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev 1997, 10 (3), 505-520.
- Wang, X.; Zhang, P.; Zhang, X., Probiotics Regulate Gut Microbiota: An Effective Method to Improve Immunity. Molecules 2021, 26 (19).
- Praveschotinunt, P.; Duraj-Thatte, A. M.; Gelfat, I.; Bahl, F.; Chou, D. B.; Joshi, N. S., Engineered E. coli Nissle 1917 for the delivery of matrix-tethered therapeutic domains to the gut. Nat Commun 2019, 10 (1), 5580.
- Cui, H.-Y.; Wang, S.-J.; Song, F.; Cheng, X.; Nan, G.; Zhao, Y.; Qian, M.-R.; Chen, X.; Li, J.-Y.; Liu, F.-L.; Zhu, Y.-M.; Tian, R.-F.; Wang, B.; Wu, B.; Zhang, Y.; Sun, X.-X.; Guo, T.; Yang, X.-M.; Zhang, H.; Li, L.; Xu, J.; Bian, H.-J.; Jiang, J.-L.; Chen, Z.-N., CD147 receptor is essential for TFF3-mediated signaling regulating colorectal cancer progression. Signal Transduct Target Ther 2021, 6 (1), 268.