Project Safety
Biosafety is an important part of project design. We primarily focus on project safety design from four aspects: biological design, production, transportation, and use.
We have also consulted relevant professionals regarding project safety. To learn more, enter Human Practices.
Design
In the project design, we designed different safety systems based on different purposes and from the levels of life. Since we expressed the temperature-sensing suicide pathway and quorum response pathway in E. coli, selected the plasmid vector lack of oriT region1, and added an overall control switch2, the engineered bacteria can only maintain normal biological activity in the human intestinal environment. At the same time, we can also stabilize the overall switch expression by applying foreign small molecule substances to achieve the effect of artificial control3. This will effectively prevent bacteria from leaking into other tissues of the human body and the external environment, while ensuring the bacterial population and MRSA response sensitivity.
Below we will elaborate on our security design.
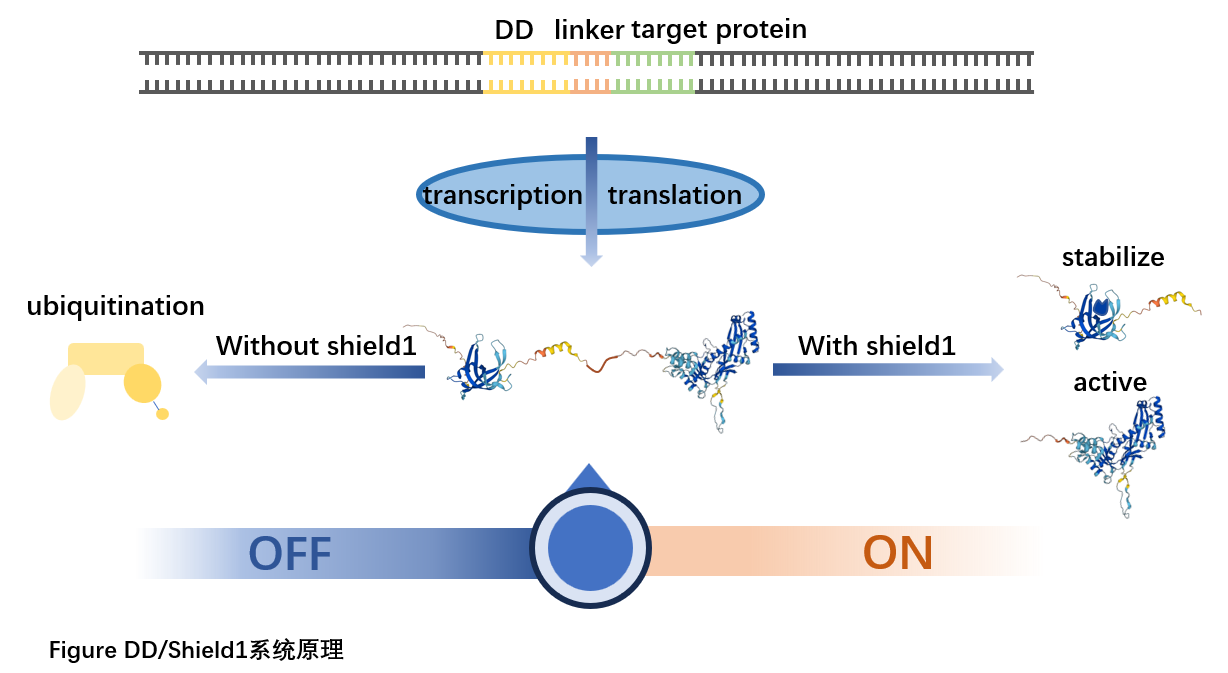
DD/Shield13: This switch consists of a dd domain and an MS2 protein that binds to the ribonucleoprotein U2-snRNP to inhibit transcription. In our design, MS2 is fused with an unstable domain (DD) via a Gly Ser adapter and is stable after administration of Shield1. Shield1 acts as a small molecule inhibitor that releases MS2 after stable DD. Free MS2 binds to U2-snRNP and inhibits the pathway, enabling the control of transcription under unknowable conditions or extreme conditions.
Plasmids can be divided into conjugative plasmids, mobilizable plasmids, and non-mobilizable plasmids. Among them, the conjugative plasmid can be autonomously transferred, including oriT, T4CP (type IV chaperone), T4SS (type IV secretion system) and relaxation enzyme genes. Mobile plasmids cannot be transferred autonomously due to the lack of one or more modules of the gene of T4CP, T4SS and relaxase, but at least contain oriT, so they can be induced to transfer with the assistance of other conjugative elements. While immobile plasmids do not contain oriT and cannot be transferred, which means that oriT is the most important factor affecting plasmid mobility.
We selected two native plasmids, pMUT1 and pMUT2. It was found by oriTfinder1 that they do not contain the oriT region, which means that the plasmids may not be metastasis.
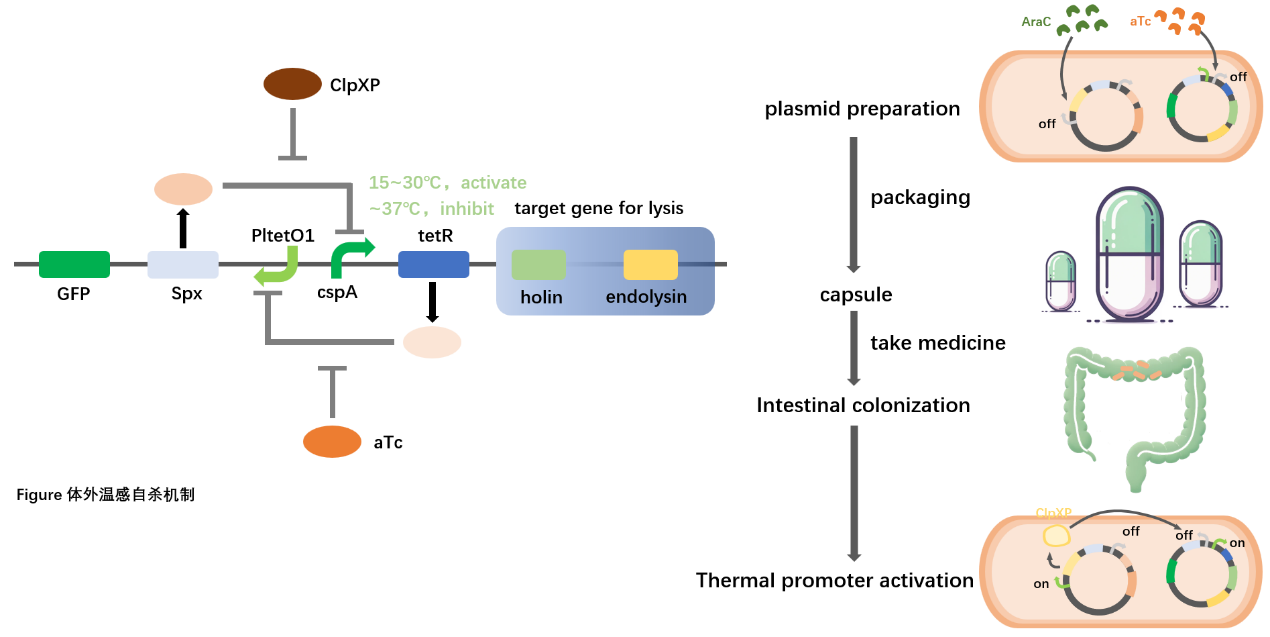
We hope that E. coli will be designed to colonize the intestinal environment for a long time, but the metabolic nature of the human body predesposes it to the risk of being excreted outside the body. To this end, we devised a genetic pathway that specifically activates in vitro and leads to E. coli suicide. We selected temperature, an environmental factor that is very different from the in vitro environment, as the initiation signal of this suicide pathway, and genes involved in cell lysis as the target genes. We use the cold shock promoter cspA derived from Staphylococcus aureus as a thermoreceptor, which has the characteristics of silent environment at 37°C and above, and specific activation at 15~37°C. So when E. coli is excreted from the body, the sudden drop in temperature activates the promoter, which triggers the expression of downstream suicide genes. At the same time, we hope that the suicide pathway will also have the following characteristics:
(1) Silence is maintained during in vitro preparation, transportation, and storage;
(2) After E. coli enters the body to regain its biological activity, the pathway needs to open up the perception of temperature.
The former guarantees that our drug will not lose its potency before it enters the body, and the latter strengthens biosecurity, as it is not possible to determine when E. coli may be excreted from the body. We meet these requirements with a dual-phase steady-state switch. Based on the cold shock promoter cspA, we constructed the above-mentioned biphasic steady state switch: the addition of the inducers ClpXP and aTc respectively can complete the transition between the suicide pathway and the green fluorescence reporter pathway. Considering that ClpXP is a protein, it is difficult to administer it exogenously, so we put it in the synthesis pathway, hoping that E. coli will be able to express itself after entering the human body.
In vitro preparation, we need to apply small molecule aTc in the environment to ensure that the suicide pathway remains silent until the drug takes effect, and at the same time, the biphasic state transition can be monitored by detecting the fluorescence reaction of the solution. The addition of Arac silences the promoters that control ClpXP in the synthesis pathway, ensuring that it does not interfere with the aforementioned conversions. Once in the human body, E. coli regains its biological activity from the dry powder state, and ClpXP is continuously expressed in the absence of aTc and Arac, and the cryogenic suicide pathway regains its sensitivity to temperature.
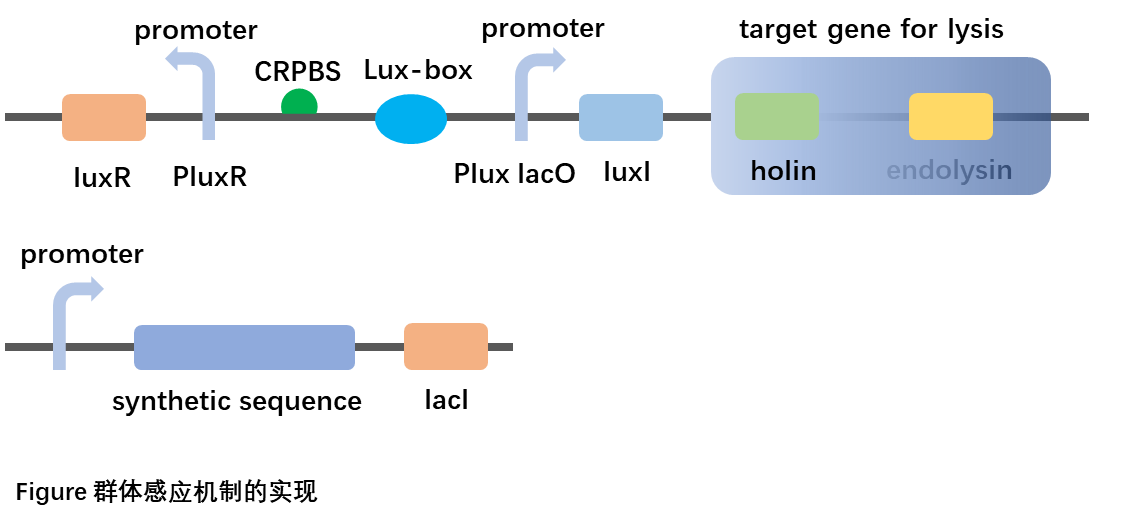
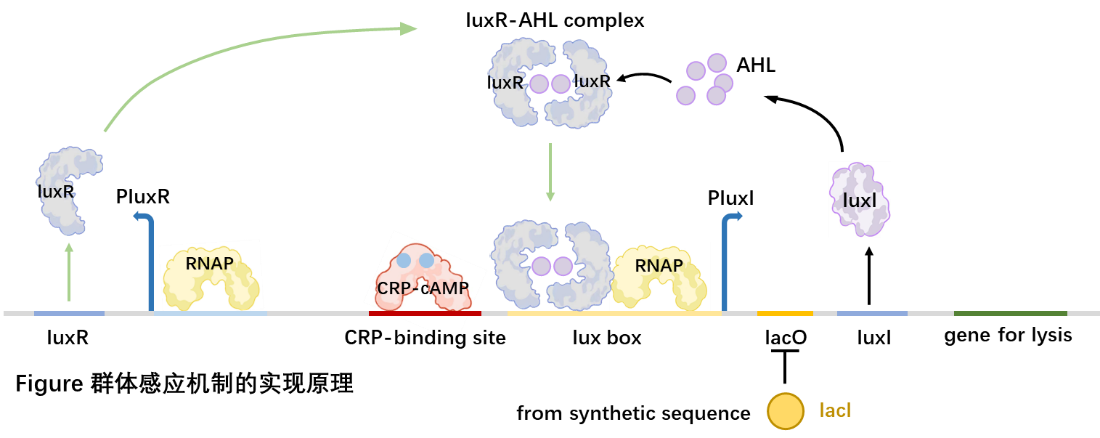
When Staphylococcus aureus is infected, the expression pathway is turned on, a large amount of LacI is expressed, which binds to LacO, inhibiting the expression of downstream genes such as LuxI, so that closing the QS system.
When Staphylococcus aureus was not infected, LacI was not expressed, LuxI was expressed. The QS system was turned on normally.
Subsequently, if we considered integrating genes into the genome of bacteria, we added an orthogonal codon system to redefine the coding rules of four amino acids, namely alanine, histidine, proline, and leucine, and thus constructed six orthogonal systems4. This makes it impossible for recombinant plasmids in E. coli to escape into other bacteria.
Production
According to our design, therapeutic-engineered bacteria will be produced in large, specialized microbial facilities. We believe that the production site has the right equipment and well-trained personnel. However, we would like to emphasize that laboratories and premises in the production process must comply with international biosafety standards and ensure a strict level of biosafety. Cultivation of E. coli must be performed in a controlled bioreactor to minimize the risk of leakage. Personnel must be specially trained and strictly follow operating procedures, including wearing protective gear and using biological safety cabinets. Waste must be treated and disposed of in accordance with biohazardous waste standards to prevent biomaterial leakage.
Transportation
We believe that the transportation process is one of the most critical aspects of biosecurity in bacteriological treatment programs. After thorough research, we determined that the use of lyophilized bacterial powder for transportation was the best choice. Lyophilized bacteria powder can be stored at room temperature for long periods of time, without the need for complex cold chain equipment, and without compromising bacterial activity and stability. In addition, such bacteria are not prone to leakage and contamination. During transportation, double-sealed containers should be used to minimize the risk of bacterial leakage. The entire transport process must be continuously monitored to ensure that no leaks or contamination occur.
Use
We use the lyophilized powder of live bacteria in enteric-coated capsules, which makes it easier and safer to take.
In the use of drugs, although many measures have been taken to ensure that viable engineered bacteria do not enter the environment. However, we still want to emphasize that the recycling of waste drugs still has to follow the norms.
Lab Safety
Laboratory safety is an essential component of the iGEM project. We have implemented various measures to ensure the biosafety of the iGEM laboratory for every participant involved.
Safety Regulations
All wet experiments are conducted at Zhejiang University Life Science Laboratory. Following the university's regulations, all experimental procedures adhere to the “Zhejiang University Laboratory Biosafety Management Measures"
All team members have undergone and passed the laboratory safety education and examination system, qualifying them for participation in wet experiments.
Safety Education
Before starting wet labs, instructors and advisors will provide detailed instructions on the proper use of laboratory equipment, emphasizing precautions when working with hazardous equipment. This safety education can give us a comprehensive understanding of the safety regulations that should be followed in the laboratory and the safety precautions that should be taken during the experiment.
Safety Protocol Agreement
Before participating in wet experiments, all team members have signed safety protocol agreements to demonstrate their understanding of laboratory safety protocols and their commitment to responsible behavior.
Daily Arrengement
In order to constantly remind everyone about safety during experiments, safety signs are posted throughout the laboratory. Specific instructions for using the equipment are posted on relevant experimental equipment, such as waste tanks for discarding specialized liquids.
We implement the "All Takes Two" system. Every detailed operation during the experiment needs to be completed by a team of two people present and collaboratively, and a confirmation letter must be signed. This ensures our daily commitment to environmental protection and safety.
Reference
- Li, X.; Xie, Y.; Liu, M.; Tai, C.; Sun, J.; Deng, Z.; Ou, H.-Y., oriTfinder: a web-based tool for the identification of origin of transfers in DNA sequences of bacterial mobile genetic elements. Nucleic Acids Res 2018, 46 (W1), W229-W234.
- Elowitz, M. B.; Leibler, S., A synthetic oscillatory network of transcriptional regulators. Nature 2000, 403 (6767), 335-338.
- Haugwitz, M.; Garachtchenko, T.; Nourzaie, O.; Gandlur, S.; Sagawa, H., Rapid, on-demand protein stabilization and destabilization using the ProteoTuner™ systems. Nature Methods 2008, 5 (10), iii-iv.
- Zürcher, J. F.; Robertson, W. E.; Kappes, T.; Petris, G.; Elliott, T. S.; Salmond, G. P. C.; Chin, J. W. J. S., Refactored genetic codes enable bidirectional genetic isolation. 2022, 378, 516 - 523.